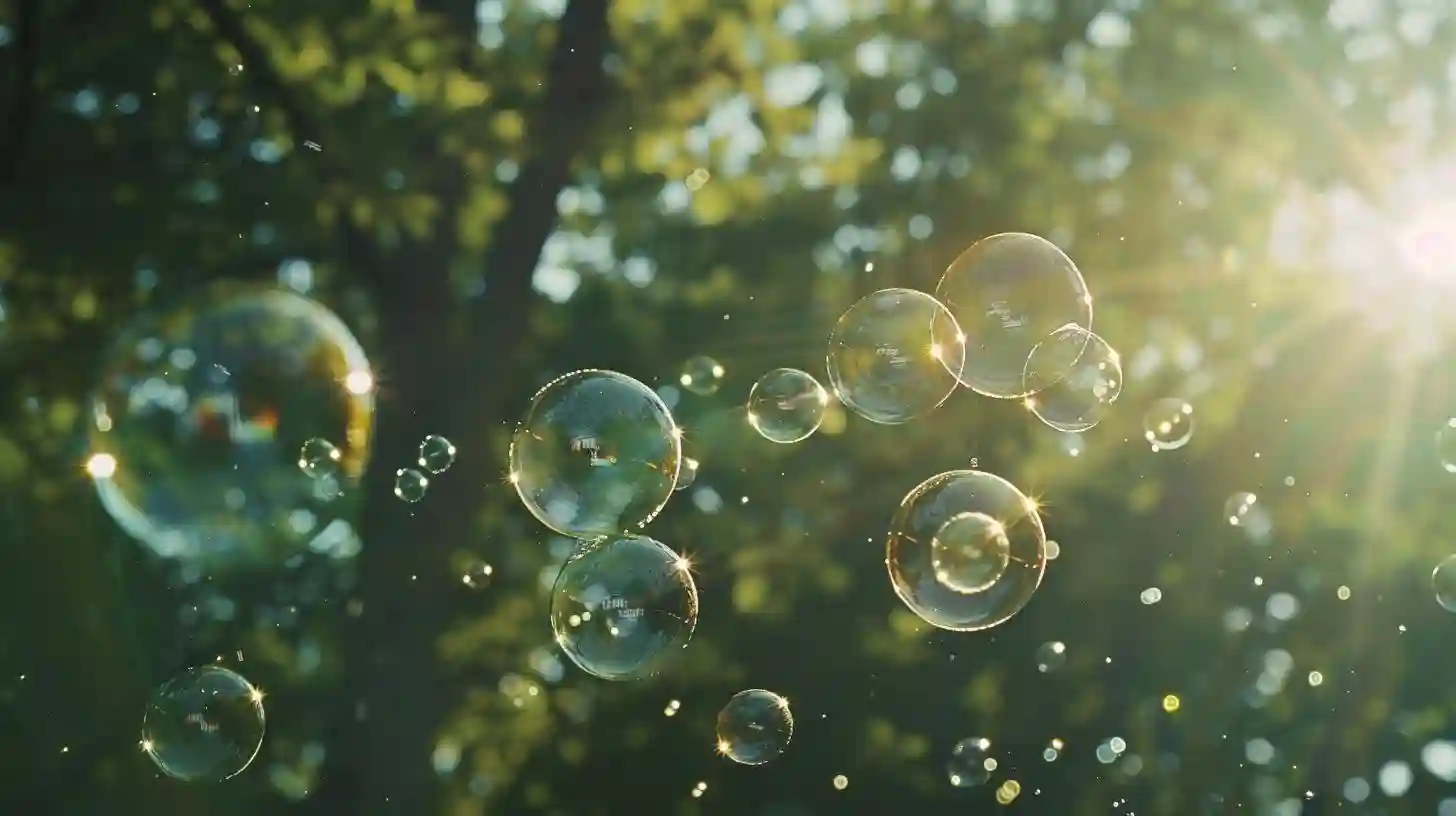
Soap bubbles have fascinated people for centuries with their delicate beauty and mesmerizing colors. These ephemeral spheres of soap and water appear almost magical as they shimmer and float through the air, catching light and reflecting rainbows. But behind their seemingly simple appearance lies a complex interplay of physics and chemistry that makes soap bubbles a fascinating subject to study.
Making bubbles starts with a simple mixture of soap and water. Soap molecules reduce the surface tension of water, allowing the liquid to spread more easily and form a thin film. When air is blown into this film, it creates a bubble, with soap molecules lining the inner and outer surfaces of the bubble to stabilize it. The thickness of the soap film and the size and shape of the bubble are critical factors in determining its stability and longevity.
The spherical shape of soap bubbles is the result of minimizing surface tension. Surface tension is the natural tendency of a liquid to minimize its surface area, which is why soap bubbles always form a sphere because it is the shape that has the smallest surface area for a given volume. This spherical shape also allows soap bubbles to be so resilient and elastic, able to stretch and deform without collapsing.
The colors of soap bubbles are another fascinating aspect of their beauty, created by the interference and diffraction of light. When light passes through a thin film of soap, some of it is reflected back and some of it passes through the film. When reflected light waves interfere with transmitted light waves, they create patterns of constructive and destructive interference, resulting in vibrant colors appearing on the surface of soap bubbles. These colors change and shift as the bubble moves and distorts, adding to the dynamic and ever-changing nature of soap bubbles.
In addition to their visual appeal, soap bubbles were also used for scientific research and experimentation. The study of soap bubbles has provided insights into surface tension, hydrodynamics and thermodynamics, as well as other areas of physics and chemistry. For example, soap bubbles have been used to study the behavior of liquids in confined spaces, as well as to model the behavior of cell membranes and other biological structures. They have also been used as a tool for teaching and demonstrating scientific principles, engaging students with their playful and interactive nature.
The playfulness of soap bubbles is perhaps one of their most enduring qualities. Children and adults alike are drawn to the simple joy of blowing bubbles, watching them float and dance through the air before bursting in a shower of tiny drops. The fragility and transience of soap bubbles serve as a reminder of the impermanence of life, encouraging us to appreciate and enjoy the beauty of the moment.
In art and literature, soap bubbles have been used as a metaphor for the transience of time and beauty. From Shakespeare's famous line in Macbeth: “Life is but a walking shadow, a poor actor who struts and worries about his hour on the stage, and then is heard no more; it is a story told by an idiot, full of sound and fury, meaning nothing,” or the paintings of Dutch artists such as Jan van Eyck and Frans Hals, who immortalized delicate bubbles in their works, soap bubbles have captured the imagination of artists and writers throughout history.
In conclusion, soap bubbles are not just objects of beauty and wonder, but also windows into the complex world of physics and chemistry. Their simple yet elegant structure conceals a complex interplay of forces and phenomena that have fascinated scientists and artists for centuries. From their production and principles to their colors and shapes, soap bubbles continue to inspire and fascinate us, reminding us of the beauty and magic that can be found in the simplest things.